Matter
    | |
| Matter is usually classified into three classical states, with plasma sometimes added as a fourth state. From top to bottom: quartz (solid), water (liquid), nitrogen dioxide (gas), and a plasma globe (plasma). |
In the classical physics observed in everyday life, matter is any substance that has mass and takes up space; this includes atoms and anything made up of these, but not other energy phenomena or waves such as light or sound. More generally, however, in (modern) physics, matter is not a fundamental concept because a universal definition of it is elusive: elementary constituents of atoms may not take up space individually, and massless particles may be composed to form objects that have mass (even when at rest).
All the everyday objects that we can bump into, touch or squeeze are ultimately composed of atoms. This ordinary atomic matter is in turn made up of interacting subatomic particles—usually a nucleus of protons and neutrons, and a cloud of orbiting electrons.Typically, science considers these composite particles matter because they have both rest mass and volume. By contrast, mass less particles, such as photons, are not considered matter, because they have neither rest mass nor volume. However, not all particles with rest mass have a classical volume, since fundamental particles such as quarks and leptons (sometimes equated with matter) are considered "point particles" with no effective size or volume. Nevertheless, quarks and leptons together make up "ordinary matter", and their interactions contribute to the effective volume of the composite particles that make up ordinary matter.
Matter exists in states (or phases): the classical solid, liquid, and gas; as well as the more exotic plasma, Bose–Einstein condensates, fermionic condensates, and quark–gluon plasma.
For much of the history of the natural sciences people have contemplated the exact nature of matter. The idea that matter was built of discrete building blocks, the so-called particulate theory of matter, was first put forward by the Greek philosophers Leucippus (~490 BC) and Democritus (~470–380 BC).
Definition
Based on mass, volume, and space
The common definition of matter is anything that has mass and volume (occupies space). For example, a car would be said to be made of matter, as it has mass and volume (occupies space).
The observation that matter occupies space goes back to antiquity. However, an explanation for why matter occupies space is recent, and is argued to be a result of the phenomenon described in the Pauli exclusion principle.Two particular examples where the exclusion principle clearly relates matter to the occupation of space are white dwarf stars and neutron stars, discussed further below.
Based on atoms
A definition of "matter" based on its physical and chemical structure is: matter is made up of atoms. As an example, deoxyribonucleic acid molecules (DNA) are matter under this definition because they are made of atoms. This definition can extend to include charged atoms and molecules, so as to include plasmas (gases of ions) and electrolytes (ionic solutions), which are not obviously included in the atoms definition. Alternatively, one can adopt the protons, neutrons, and electrons definition.
Based on protons, neutrons and electrons
A definition of "matter" more fine-scale than the atoms and molecules definition is: matter is made up of what atoms and molecules are made of, meaning anything made of positively charged protons, neutral neutrons, and negatively charged electrons.This definition goes beyond atoms and molecules, however, to include substances made from these building blocks that are not simply atoms or molecules, for example white dwarf matter—typically, carbon and oxygen nuclei in a sea of degenerate electrons. At a microscopic level, the constituent "particles" of matter such as protons, neutrons, and electrons obey the laws of quantum mechanics and exhibit wave–particle duality. At an even deeper level, protons and neutrons are made up of quarks and the force fields (gluons) that bind them together.
Matter can be classified by its state.
- Solids have a set volume and shape.The inter molecular force of attraction for solid matter is very strong.
- Liquids have a set volume, but change shape. The inter molecular force of attraction for liquid matter is weaker than solid matter.
- Gases have neither definite volume nor shape. The inter molecular force of attraction for gaseous matter is negligible.
- Plasma which are usually gaseous state of matter in which a part or all of the atoms or molecules are dissociated to form ions.
Matter can also be classified by its chemical composition.
- An element is a pure substance made up of atoms with the same number of protons. As of 2011, 118 elements have been observed, 92 of which occur naturally. Carbon (C), Oxygen (O), Hydrogen (H) are examples of elements. The periodic table is a tabular representation of the known elements.
- A compound consists of two or more chemical elements that are chemically bonded together. Water (H2O) and table sugar (C12H22O11) are examples of chemical compounds. The ratio of the elements in a compound is always the same. For example in water, the number of H atoms is always twice the number of O atoms.
- A mixture consists of two or more substances (element or compound) mixed together without any chemical bond. Salad is a good example. A mixture can be separated into its individual components by mechanical means.
Atoms & Molecule
Atom
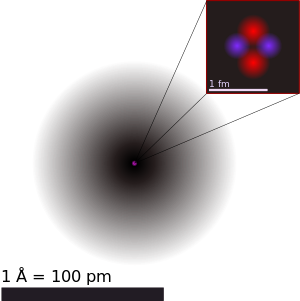
| Helium atom | ||||||||
|---|---|---|---|---|---|---|---|---|
| An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10−10 m or 100 pm). | ||||||||
| Classification | ||||||||
| ||||||||
| Properties | ||||||||
|
An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are very small; typical sizes are around 100 picometers (a ten-billionth of a meter, in the short scale).
Atoms are small enough that attempting to predict their behavior using classical physics - as if they were billiard balls, for example - gives noticeably incorrect predictions due to quantum effects. Through the development of physics, atomic models have incorporated quantum principles to better explain and predict the behavior.
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and typically a similar number of neutrons. Protons and neutrons are called nucleons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, that atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
The electrons of an atom are attracted to the protons in an atomic nucleus by this electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by a different force, the nuclear force, which is usually stronger than the electromagnetic force repelling the positively charged protons from one another. Under certain circumstances the repelling electromagnetic force becomes stronger than the nuclear force, and nucleons can be ejected from the nucleus, leaving behind a different element: nuclear decay resulting in nuclear transmutation.
The number of protons in the nucleus defines to what chemical element the atom belongs: for example, all copper atoms contain 29 protons. The number of neutrons defines the isotope of the element. The number of electrons influences the magnetic properties of an atom. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature, and is the subject of the discipline of chemistry.
Molecule
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. According to this definition, noble gas atoms are considered molecules as they are in fact monoatomic molecules.
A molecule may be homonuclear, that is, it consists of atoms of one chemical element, as with oxygen (O2); or it may be heteronuclear, a chemical compound composed of more than one element, as with water (H2O). Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are generally not considered single molecules.
Molecules as components of matter are common in organic substances (and therefore biochemistry). They also make up most of the oceans and atmosphere. However, the majority of familiar solid substances on Earth, including most of the minerals that make up the crust, mantle, and core of the Earth, contain many chemical bonds, but are not made of identifiable molecules. Also, no typical molecule can be defined for ionic crystals (salts) and covalent crystals (network solids), although these are often composed of repeating unit cells that extend either in a plane (such as in graphene) or three-dimensionally (such as in diamond, quartz, or sodium chloride). The theme of repeated unit-cellular-structure also holds for most condensed phases with metallic bonding, which means that solid metals are also not made of molecules. In glasses (solids that exist in a vitreous disordered state), atoms may also be held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating units that characterizes crystals.
Properties of Matter
Intensive and extensive properties
Intensive properties
An intensive property is a physical quantity whose value does not depend on the amount of the substance for which it is measured. For example, the temperature of a system in thermal equilibrium is the same as the temperature of any part of it. If the system is divided the temperature of each subsystem is identical. The same applies to the density of a homogeneous system; if the system is divided in half, the mass and the volume change in the identical ratio and the density remains unchanged. Additionally, the boiling point of a substance is another example of an intensive property. For example, the boiling point of water is 100 °C at a pressure of one atmosphere, which remains true regardless of quantity.
The distinction between intensive and extensive properties has some theoretical uses. For example, in thermodynamics, according to the state postulate, a sufficiently simple system consisting of a single substance requires only two independent intensive variables to fully specify the system's entire state. Other intensive properties are derived from those two variables.
Examples
Examples of intensive properties include:
- chemical potential, μ
- color[6]
- concentration, c
- density, ρ (or specific gravity)
- magnetic permeability, μ
- melting point and boiling point[7]
- molality, m or b
- pressure, p
- specific heat capacity, cp
- specific volume, v
- standard reduction potential,[7] E°
- temperature, T
Extensive properties
The IUPAC Gold Book defines an extensive property as a physical quantity whose magnitude is additive for subsystems.The value of such an additive property is proportional to the size of the system it describes, or to the quantity of matter in the system. For example, the amount of heat required to melt ice at constant temperature and pressure is an extensive property, known as the enthalpy of fusion. The amount of heat required to melt one ice cube would be much less than the amount of heat required to melt an iceberg, so it is dependent on the quantity.
Extensive properties are not just dependent on the amount of material in a system; the relation must be additive. If, say, a property depended on the square of the mass, it would not be an extensive property. (Consider a system consisting of two 1 gram weights. The total mass is 2 g, squaring that gives 4 g2. Squaring and summing the individual masses gives 2 g2. This property is not additive for the two subsystems.)
Dividing one extensive property by another extensive property generally gives an intensive value—for example: mass (extensive) divided by volume (extensive) gives density (intensive).
Examples
Examples of extensive properties include:
- amount of substance, mol
- energy, E
- enthalpy, H
- entropy, S
- Gibbs energy, G
- heat capacity, Cp
- Helmholtz energy, A or F
- internal energy, U
- mass, m
- volume, V